Absolute zero and the third law of thermodynamics
The absolute zero is referring to a temperature of zero degrees on the Kelvin
scale or thermodynamic scale. It is written as 0 K and read as zero Kelvin.
It is equivalent to -273.15 degrees Celsius or -459.67 degrees Fahrenheit.
The third law of thermodynamics states that the absolute zero is the coldest temperature possible!
Now, why do we say that it cannot get any colder than this?
Kinetic energy can help us understand this concept because temperature depends on the kinetic energy of the atoms of a substance.
There seems to be no limit to how much you can increase the temperature of a substance.
The more heat you put into a substance or something, the higher the kinetic energy of the atoms, and the more agitated the atoms or molecules will be in that substance.
However, can we always decrease the temperature of a substance?
No way! When we decrease the movement of atoms in a substance, we are decreasing the kinetic energy of these atoms.
At the same time, the temperature will decrease as well.
However, once the kinetic energy reaches zero, these atoms don't move anymore.
Since they don't move, they have no energy anymore and it does not make sense to say you will extract more energy from them.
Since you cannot extract more energy, you cannot lower the temperature any further.
We have then reached your lowest temperature!
Absolute zero and the Kelvin scale
Now that you know what absolute zero is, check out the kelvin scale below showing some absolute temperatures. British physicist Lord Kevin suggested this scale and it was of course named after him.
What do we say absolute temperature? It is because absolute zero was used as a reference point to measure these temperatures.
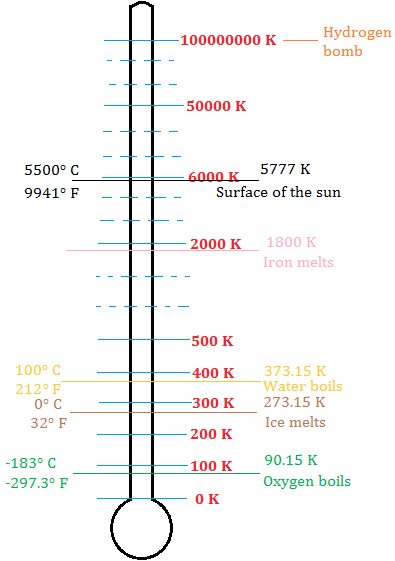
Why do we use the Kelvin scale?
- The Kelvin scale does not use negative number, so it is easier for scientists to do math calculation when the temperature is very low.
- The Kelvin scale is frequently used in thermodynamics.
- The Kelvin scale is also used to give credit to Lord Kevin who suggested it.