What is density in physics?
What is density? Mathematically speaking, density is the ratio of the
mass to the volume. Physically speaking, density is the measure of how
much matter is squeezed in a given space.
The more matter is squeezed in the same space, the more dense.
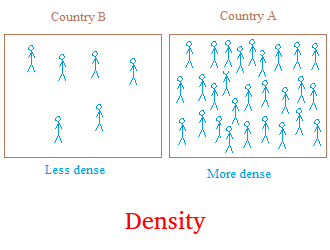
Looking at the figure above, you may have heard people saying that the population of country A is
very dense. Well, this means that compare to country B that has about the same surface area as country A, country A
has a lot
more people living in it than country B.
Another good example of density is a sponge. Take that same sponge and squeeze the whole thing until the sponge becomes very small.
Since the volume of the sponge is much less after you have squeezed it, the sponge has become more dense. You are able to put the same amount of matter in a smaller space or volume.
Let us see why this makes sense using the formula for density.
Pretend that the volume of the sponge is 80 cm3
Pretend that the mass is 10 grams. Notice that these are very good estimate for the mass and volume of a sponge.
density = 0.125 g/cm3
Suppose that after squeezing the sponge the volume is now 4 times less such as 20 cm3
density = 0.50 g/cm3
Since 0.50 g/cm3 is bigger than 0.125 g/cm3, the sponge is more dense when you squeeze it.
You can also increase the density by increasing the mass. Say the mass of another sponge is now 40 grams and you are able to squeeze this sponge with your hand so hard until the volume is 20 cm3
density = 1.50 g/cm3
As you can see now, 1.50 grams, or a lot more mass, is squeezed in the same space or volume.
The density is determined by both the masses of atoms and the space between atoms.
In other words. The higher the mass, the higher the density. The less space between atoms, the higher the density.
The densest substance on earth is the osmium. The density is 22.6 g/cm3
What is density of some other
materials?
A list is given below for some materials. Keep in mind that density varies somewhat with pressure and temperature.
With the exception of water, density is given at 0°C and atmospheric pressure.Solid materials Density (g/cm3)
Platinum 21.4
Gold 19.3
Lead 11.3
Silver 10.5
Copper 8.9
Iron 7.8
Steel 7.8
Diamonds 3.5
Aluminum 2.7
Graphite 2.25
Ice 0.92
Pine wood 0.50
Liquid materials Density (g/cm3)
Mercury 13.6
Seawater 1.03
Water at 4°C 1.00
Ethyl alcohol 0.81
Definition of weight density
Weight density is the ratio of the amount of weight a body has to the volume.
Definition of specific gravity
Specific gravity is the ratio of the density of a material to the density of water.
g/cm3 is on top and at the bottom, so it will cancel.
Weight density of gold = 19.3. The specific gravity of every material
above is therefore, the number that you see without the unit.